Dissociation of Water Molecules
Part A

Correct
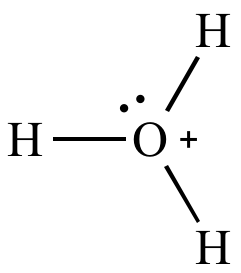
A water molecule that gains a hydrogen becomes a hydronium ion.
Part B

Correct
This molecule was formed by the transfer of a hydrogen ion from one water molecule to another.
Part C

Correct
A water molecule that loses a hydrogen ion is referred to as a hydroxide ion.
Part D
To view the animation, click here. Then click on the image to start the animation.
Correct
The transfer of a hydrogen atom from one water molecule to another is referred to as dissociation.
Part E
Correct
This is the correct equation for the dissociation of water molecules.
Part F
Correct
The formation of hydronium results in the gain of 1 more proton than electron.
Part G
Correct
A hydroxide ion is formed when a water molecule loses a hydrogen ion.
Part H
Correct
One in half a billion.

